The aim of the project is to make novel durable catalyst support materials for HT-PEMFC. The focus is on the synthesis and physiochemical as well as electrochemical characterisation of SiC with different morphologies.
By using shape memory synthesis, SMS, with different carbon templates, it is possible to exploit the different morphologies of the template to form SiC samples with different physiochemical properties. SMS is the reaction of carbon with in situ formed SiO gas. The carbon template is put in a tube furnace downstream to a well-mixed stack of Si and SiO2. Under an argon flow the reactants are heated to 1475°C for 15-20 hours. During the synthesis, Si from SiO gas, is substituting every second C atom in the carbon template and producing CO gas. The name of the synthesis relates to the macroscopic scale, where the produced SiC have the same shape as the carbon template.
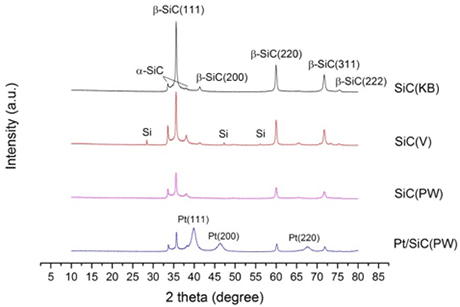
Figure 1: PXRD patterns of SiC prepared from respectively Ketjenblack (KB), Vulcan (V) and pyrolysed wood (PW) and of the catalyst Pt/SiC(PW). All products consist of both α- and β-SiC. The PXRD of Pt/SiC(PW) confirms that Pt is successfully added, the β-SiC(200) peak is hidden by the Pt(111) peak. SiC(V) is contaminated with Si from the unreacted reactants
Synthesis conditions and facilities have been optimised and SiC has been synthesised through SMS from three different carbon templates: pyrolysed wood, Vulcan and Ketjen Black. Powder X-Ray Diffraction, PXRD, of the products reveal the presence of both α- and β-SiC in all samples, see figure 1. Excess carbon was removed by phase separation and heating and the products compared by PXRD and particle size distribution. The heat treatment was chosen due to more small particles. Pt was added by the polyol method to an acid treated sample of SiC. The presence of Pt is confirmed by PXRD, see figure 1, which show characteristic Pt peaks, and by cyclic voltammetry, see figure 2, which show the characteristics of Pt.
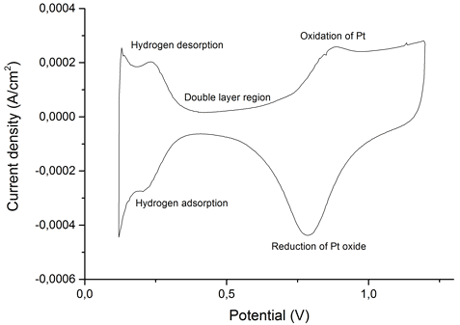
Figure 2: CV of Pt/SiC(PW) in 0.1 M HClO4, 0.05 V – 1.2 V, 50 mV/s. The CV has the characteristic desorption and adsorption of hydrogen as well as the oxidation and reduction of Pt confirming that Pt is present.